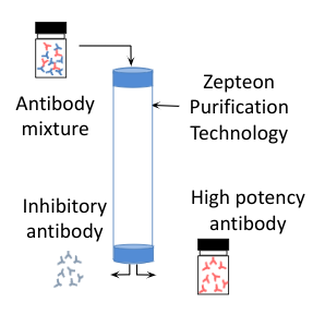
Technology.Zepteon has developed Glycap-3A, a unique and patented (US10221210B2, EP2734537A1) affinity purification technology that enables separation of antibodies based on their glycoform (nonfucyosylated or bisected GlcNAc) and subclass (high affinity for IgG1 and IgG3). The affinity purification resin contains optimally immobilized FcgammaR3A receptors, leading to high antibody binding capacities. The FcgammaR3A is glycosylated and represents a superior technology compared to competitors non-glycosylated E. coli derived FcgammaR3A ligands which have limited selectivity.
|
New mAb and IVIG TherapiesUsing approved antibodies as starting materials such as monoclonal antibodies or plasma derived intravenous immunoglobulin (IVIG), drug developers can rapidly assess the impact of antibody glycoforms and subclass with in vitro or in vivo function.
|
Biosimilars DevelopmentRegulatory agencies require that biosimilars developers closely match innovator molecules in terms of glycosylation and structure:function. Glycap-3A purification can finely tune the level fucose in a biosimilar antibody and be used to thoroughly analyze structure function relationships.
|
Hyperimmune anti-viral IgGPlasma derived intravenous immunoglobulin (IVIG) is currently used to treat a number of diseases including virus infections, autoimmunity and for replacement therapies. Leveraging Glycap-3A, new therapies can be developed to enrich or deplete immune activating glycoforms or subclasses as desired.
|
New mAb and IVIG Therapies
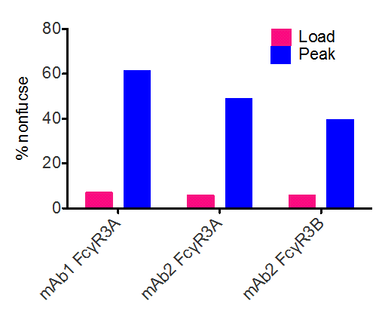
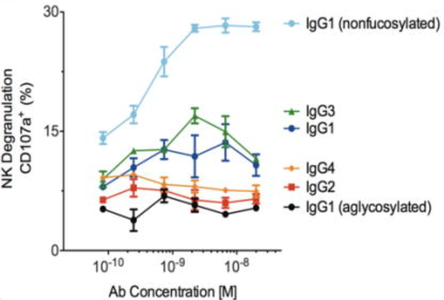
Glycap-3A uses immobilized Fc gamma R3A IgG receptor that readily binds to nonfucosylated, bisected GlcNAc, IgG1 and IgG3 subclasses. When developing novel mAb or IVIG therapeutics, understanding the structure:function relationship between glycans or subclasses and in vitro or in vivo function is valuable and can be useful for developing the mechanism of action (MOA) for regulatory filings. We've previously published using Fc gamma receptors for both monoclonal mAb characterization where show both FcgR3A and 3B receptors enrich in nonfucosylated mAbs (Bolton et al. 2013) and IVIG structure:function studies that show nonfucosylated and bisected GlcNAcs on IgG1 and IgG3 have the most potent effector functions in cellular assays (Boesch et al. 2018).
Biosimilars Development
The problem biosimilar monocloncal antibody makers have to solve is matching the properties of the original drug. During manufacturing, antibodies are produced in cells that attach various sugar structures like fucose. The FDA and EMA have indicated that biosimilar antibody manufacturers should compare receptor binding of their candidate to that of the innovator drug. Biosimilar drugs with equal nonfucosylation levels as those of the innovator drugs would have similar receptor binding and therefore be likely to gain regulatory approval. The Zepteon Glycap-3A, affinity purification technology that addresses this critical need for biosimilars makers by enabling separation of antibodies based on their fucose levels.
Zepteon's Glycap-3A resin can be used to make a biosimilar monocloncal antibody with a nonfucose level that matches that of the original drug, or one with a nonfucose level at the upper end of the range of the original drug for use in structure function studies (Pace et. al, Biotech Prog, June 2016; btpr.2300). The economical, fast and robust Zepteon separation method is superior to cellular methods of targeting fucose levels. Cellular methods like genetically engineering cells or screening cell lines and bioreactor conditions are slow, complex and costly. The glycoforms obtained using the Zepteon resin are the most physiologically relevant since the separation is performed using natural human receptors.
Zepteon's Glycap-3A resin can be used to make a biosimilar monocloncal antibody with a nonfucose level that matches that of the original drug, or one with a nonfucose level at the upper end of the range of the original drug for use in structure function studies (Pace et. al, Biotech Prog, June 2016; btpr.2300). The economical, fast and robust Zepteon separation method is superior to cellular methods of targeting fucose levels. Cellular methods like genetically engineering cells or screening cell lines and bioreactor conditions are slow, complex and costly. The glycoforms obtained using the Zepteon resin are the most physiologically relevant since the separation is performed using natural human receptors.
Hyperimmune anti-viral IgG
Hyperimmune IgG is typically obtained from human donors who have been exposed to a virus and have generated virus specific antibodies. Literature indicates hyperimmune IgG would be far more potent against a range of viruses (Flu, MERS-CoV, Ebola, HPV, West Nile, CHIKV) using optimal IgG glycoforms and subclasses (Zeitlin et. al, PNAS, 2011; doi: 10.1073/pnas.1108360108).
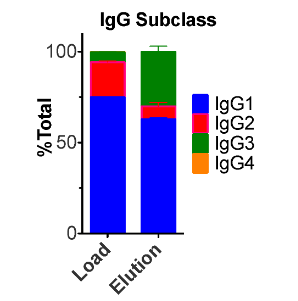
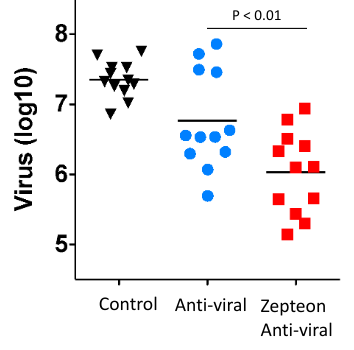
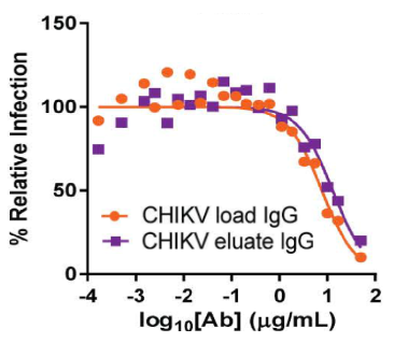
Anti-viral plasma IgG fractionated using the Glycap-3A resin has been demonstrated to enhance the potency of Anti-viral hyperimmune IgG 8-fold in vivo by enriching for the highest potency glycoforms and removing inhibitory IgG2 and IgG4. The figure below shows the reduction in viral count in mice in a Chikungunya (CHIKV) post-exposure study with the Zepteon Glycap-3A enriched anti-viral hyperimmune IgG (eluant) compared to the starting material prior to purification (load). Additionally, the in vitro viral neutralization potency of the hyperimmune IgG was not altered by the purification method indicating the enhanced anti-viral potency was improved by enhancing the immune cell engagement and effector functions via Fc receptors due to the subclass and glycan enrichment from Glycap-3A purification.
Anti-viral plasma IgG fractionated using the Glycap-3A resin has been demonstrated to enhance the potency of Anti-viral hyperimmune IgG 8-fold in vivo by enriching for the highest potency glycoforms and removing inhibitory IgG2 and IgG4. The figure below shows the reduction in viral count in mice in a Chikungunya (CHIKV) post-exposure study with the Zepteon Glycap-3A enriched anti-viral hyperimmune IgG (eluant) compared to the starting material prior to purification (load). Additionally, the in vitro viral neutralization potency of the hyperimmune IgG was not altered by the purification method indicating the enhanced anti-viral potency was improved by enhancing the immune cell engagement and effector functions via Fc receptors due to the subclass and glycan enrichment from Glycap-3A purification.
Julie Fox et al. Keystone: Antibodies as Drugs. Whistler, Feb 2018